On August 4, 2020, the Centers for Medicare & Medicaid Services (CMS) released the Changes to Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; Addition of New Categories for Hospital Outpatient Department Prior Authorization Process [CMS-1736-P], which includes proposed payment updates and policy changes affecting Medicare hospital outpatient and ambulatory surgical center (ASC) services for CY 2021. The proposed regulations will be published in the Federal Register on August 12, 2020. Comments are due October 5, 2020.
For CY 2021, CMS proposes to increase payment rates under the Hospital Outpatient Prospective Payment System (OPPS) and the ASC Payment Systems by a factor of 2.6%. In continuation of an existing policy, hospitals and ASCs that fail to meet their respective quality reporting program requirements are subject to a 2.0% reduction in the CY 2021 fee schedule increase factor.
CMS estimates, based on the proposed policy changes, that total payments to hospitals and ASCs will be approximately $83.9 billion and $5.45 billion, respectively, for an increase of approximately $7.5 billion and $160 million, respectively, over CY 2020 program payments.
CMS indicates that they are waiving the 60-day publication requirement for the Final Rule and replacing it with a 30-day notification, meaning the Final Rule will be effective January 1, 2021, even though it may not be published until December 1, instead of the typical November 1 target publication date.
Read on for a topline summary of the major provisions in the proposed rule.
Payment for 340B Drugs
Key Takeaway: CMS proposes to pay ASP minus 28.7% for 340B drugs.
For CY 2018, CMS implemented a controversial change whereby Medicare pays for drugs covered and paid under the OPPS and purchased through the 340B Program at Average Sales Price (ASP) minus 22.5%, instead of the traditional ASP plus 6%. For CY 2019, CMS extended this policy by also paying ASP minus 22.5% for 340B drugs furnished by non-excepted off-campus provider-based departments. CMS maintained the policy for CY 2020 despite ongoing litigation and several court rulings that CMS exceeded its authority when it implemented these policy changes. Notably, on July 31, 2020, the US Court of Appeals for the District of Columbia Circuit held that CMS does have authority under the Social Security Act to reduce Medicare payment rates for 340B-eligible drugs reimbursed under the OPPS.
For CY 2021, CMS proposes to further revise current payment policy for 340B-eligible drugs. Specifically, CMS proposes to utilize data on drug acquisition costs in CYs 2018 and 2019 from the Hospital Acquisition Cost Survey for 340B-Acquired Specified Covered Drugs to establish payment for CY 2021 and beyond.
According to CMS, ASP minus 22.5% was a conservative estimate of covered entity drug acquisition costs, and current policy overcompensates covered entity hospitals for drugs acquired under the 340B program. As a result, for CY 2021, CMS proposes to set payment for 340B-acquired drugs by reducing ASP by the volume-weighted geometric mean discount, excluding “penny priced drugs”—or drugs where the 340B Ceiling Price falls below $0—and statistical outliers. CMS now proposes to pay for drugs purchased through the 340B program at ASP minus 34.7%, plus an add-on of 6% of ASP to cover overhead and handling costs, for a net proposed rate of ASP minus 28.7%.
At the time of the proposed rule’s publication, CMS had not made public the 340B survey data and even if released will not include data on 340B Ceiling Prices due to statutory confidentiality provisions. Therefore, stakeholders are unable to validate the CMS analysis and calculation methodology.
CMS is holding out the prospect of delaying the cut. The agency solicits comments on an “alternative” proposal under which it would continue the current Medicare payment policy of ASP minus 22.5% for 340B drugs paid under OPPS.
As in past years, rural sole community hospitals, children’s hospitals and prospective payment system-exempt cancer hospitals would be exempt from payment cuts to 340B drugs and would continue to be paid at ASP plus 6%. Similarly, as in past years, the 340B payment cuts would be implemented in a “budget neutral” manner, with the amounts of the payment cuts being used to increase OPPS payments on all other services to all hospitals paid under OPPS.
Revisions to the Inpatient Only List
Key Takeaway: CMS proposes to eliminate the inpatient only list over a three-year period.
Historically, CMS has identified services that are typically provided in an inpatient setting (referred to as the inpatient only (IPO) list) and that thus would not paid by Medicare under the OPPS. For CY 2020, there are 1,740 services on this list. Each year, as part of its annual review, CMS seeks to identify services that should be removed from or added to the list based on the most recent data and medical evidence available.
For many years, CMS has received comments, mostly from surgeons and surgical centers, requesting that CMS eliminate the IPO list and allow physicians to use clinical judgment to determine site of service. Generally, the hospital community has objected to any such change.
For CY 2021, CMS proposes to eliminate the IPO list over a three-year transition period, with completion by January 1, 2024. In its discussion of the proposed change, CMS noted several safeguards to ensure beneficiary safety in the absence of the IPO list, including:
As part of the three-year transition plan, CMS proposes to remove 266 musculoskeletal-related services from the IPO list. The list of these services can be found in Table 31 of the proposed rule. CMS seeks comments from stakeholders regarding:
Physician-Owned Hospitals
Key Takeaway: CMS proposes changes to the expansion exception process for a subset of physician-owned hospitals.
Under the federal physician self-referral proscription, commonly known as the Stark Law, a physician is prohibited from making referrals for certain specified services to any entity in which the physician (or an immediate family member of the physician) has a financial relationship (including a direct or indirect ownership or investment interest). Penalties for violating the law include steep civil monetary penalties and possible exclusion from the Medicare and Medicaid programs. Despite this self-referral prohibition, until 2010, physicians could make referrals to hospitals that they own through exceptions to the law. In 2010, after nearly a decade of rapid growth in both physician-owned hospitals and corresponding policy scrutiny, Congress enacted provisions in the Affordable Care Act severely restricting new physician-owned hospitals and limiting growth of existing facilities.
In the CY 2021 proposed rule, CMS proposes to remove certain expansion limits for “high Medicaid facilities” as part of its Patients over Paperwork Initiative. CMS proposes the following flexibilities applicable only to qualifying Medicaid facilities:
A hospital qualifies as a “high Medicaid” facility when a hospital:
CMS solicits comments on whether it should maintain the opportunity for community input on any requests for exceptions to expansion process submitted by “high Medicaid facilities”.
Prior Authorization Process for Certain Services
Key Takeaway: CMS proposes to expand the prior authorization process to include two new categories of services reimbursed under the OPPS.
For CY 2020, CMS finalized a proposal to establish a process through which hospitals must submit a prior authorization request for a provisional affirmation of coverage before a covered outpatient service is furnished to the beneficiary and before the claim is submitted for processing. The change applied to five categories of services: blepharoplasty, botulinum toxin injections, panniculectomy, rhinoplasty and vein ablation.
For CY 2021, CMS proposes to include two new categories of services—cervical fusion with disc removal and implanted spinal neurostimulators—under the prior authorization process. Services in these two categories would be subject to prior authorization for dates of service on or after July 1, 2021.
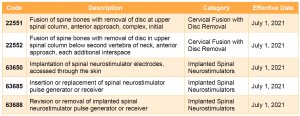
For more information on the Outpatient Department Prior Authorization process, please review our summary of the CY 2020 OPPS/ASC final rule. Prior authorization has been at the forefront of the policy debate on Capitol Hill and with the Administration, with proposals to both curtail and expand the use of prior authorization. COVID-19 has disrupted some of the focus on this issue and has simultaneously shed light on prior authorization in new ways. Stakeholders should continue to monitor the legislative and regulatory landscape for developments, in addition to those contained in this proposed rule.
Laboratory Date of Service Policy
Key Takeaway: CMS proposes to expand the date of service exception to selected multianalyte assays with algorithm analyses (MAAAs).
The date of service for a laboratory test may affect payment for patients seen in a hospital outpatient department, because if the date of service falls during an outpatient encounter, payment for the laboratory test usually is bundled with the hospital service.
For most laboratory tests, the date of service is typically the date of specimen collection, unless the specimen was archived (held for more than 30 days) before testing, in which case the date of testing is the date of service. In prior rulemakings, CMS developed exceptions to this date of service policy for some tests. Specifically, an exception was made for molecular diagnostic tests and certain tests that have obtained advanced diagnostic laboratory test (ADLT) status if:
Currently, the date of service exception does not apply to protein MAAA tests unless the assay had obtained ADLT status. For CY 2021, CMS proposes to extend the laboratory date of service exception to cancer-related protein MAAA tests. The proposed rule notes that this change will affect tests described by five CPT codes (81500, 81503, 81535, 81536 and 81539). CPT code 81538, which is also a cancer-related protein MAAA, is currently recognized as an ADLT and thus is already eligible for the date of service exception. CMS is not proposing to include non-cancer-related protein MAAA tests to this expanded exception.
Transitional Pass-Through Payment for Medical Devices
Key Takeaway: CMS solicits comments on providing separate payment following the end of the pass-through period for medical devices with reduced utilization during the public health emergency.
During the current public health emergency, volume for some services, as well as devices used in services that may be considered elective, has been down. Medical devices are only eligible for transitional pass-through payments for a maximum of three years. Currently, seven device categories are eligible for transitional pass-through payment. In response to concerns expressed by stakeholders, CMS requests comments on using its authority to provide separate payment for an undefined period of time after pass-through status ends for these device categories to account for the period of time that device utilization was reduced.
CMS does not propose any changes to its qualification criteria for transitional pass-through payments for medical devices. As part of this review cycle, CMS evaluated five applications for device pass-through payments and preliminarily approved two—CUSTOMFLEX® ARTIFICIALIRIS and EXALT™ Model D Single-Use Duodenoscope.
Site-Neutral Payments for Clinic Visits at Off-Campus Provider-Based Departments
Key Takeaway: CMS will continue to pay clinic visits provided by off-campus hospital outpatient departments at 40% of the OPPS rate.
Beginning in 2019, CMS implemented a policy that reduced OPPS payments for clinic visits described by HCPCS code G0463 and furnished at off-campus provider-based outpatient departments that previously were excepted or grandfathered from site-neutral payment policies. CMS phased in the payment reduction over two years. For 2020, CMS implemented the second portion of the payment reduction, a change that reduced payments for these services to 40% of the OPPS rate.
This change is controversial and is the subject of litigation. In September 2019, a federal district court sided with hospital plaintiffs, ruling that CMS lacked statutory authority to implement the change. However, on July 17, 2020, the US Court of Appeals for the District of Columbia Circuit ruled in favor of CMS, holding that the agency’s regulation was a reasonable interpretation of the statutory authority to adopt a method to control for unnecessary increases in the volume of the relevant service.
In light of this recent court victory, CMS will continue this policy in 2021. CMS has not released information on how or if it will address reprocessing 2019 claims that were previously reprocessed at the higher OPPS rate.
ASC Covered Procedures List
Key Takeaway: CMS proposes a new process for identifying services to be added to the ASC covered procedures list (CPL).
Similar to the IPO list, CMS reviews the ASC CPL annually to determine if there are services that should be added to or removed from the list. Changes to the list are often driven by stakeholder feedback.
The COVID-19 pandemic has had a significant impact on healthcare facilities and has highlighted the need for additional healthcare access points for beneficiaries across the country. Many ASCs have either temporarily or permanently closed, or temporarily enrolled as hospitals during the public health emergency. To ensure continued access to care during and after the PHE, CMS is seeking to give physicians and patients greater flexibility to choose ASCs as a site of care for covered surgical procedures.
To further that goal, CMS proposes to add 11 procedures to the ASC CPL, including total hip arthroplasty. A full list of codes can be found at Table 40 of the proposed rule.
Most significantly, CMS seeks comments on two alternative processes for identifying procedures to be added to the ASC CPL.
Alternative 1 – Nomination Process. Stakeholders (including medical societies) would nominate procedures for consideration to be added to the ASC CPL. Any procedures recommended to CMS would be reviewed as part of the annual rulemaking cycle, with CMS summarizing the recommendations and the justification for inclusion or exclusion on the list. Comments would be submitted as part of the annual rulemaking cycle, with final decisions published in each year’s final rule. CMS would also modify a subset of the criteria used to evaluate the inclusion of a service on the ASC CPL. Under this proposal, the nomination process would begin in CY 2021 for CY 2022.
Alternative 2 – Revision of Exclusion Criteria. CMS would use the existing process for annually reviewing services for consideration but CMS would eliminate the following five of the current eight general exclusion criteria for adding surgical procedures to the ASC CPL:
Historically, CMS has excluded procedures if the procedure requires inpatient care and thus sits on the IPO list. With the proposed elimination of the IPO list over a three-year period, CMS proposes to exclude procedures designated as requiring inpatient care as of December 31, 2020. With the proposed alternative two, CMS could implement the change for CY 2021, rather than CY 2022. Under this approach, CMS identified 270 procedures that could be added to the ASC CPL. The full list of potential codes is listed in Table 41 of the proposed rule.
Overall Star Quality Ratings
Key Takeaway: CMS proposes updates to the Overall Quality Star Ratings.
The Overall Quality Star Ratings summarize a variety of quality measures on Hospital Compare. The star ratings range from one to five and are based on data from the Hospital Inpatient Quality Reporting Program and the Hospital Outpatient Quality Reporting (OQR) Program.
For CY 2021 and subsequent years, CMS proposes several changes to simplify the methodology used to calculate ratings, reduce provider burden, improve the predictability of the star ratings and increase the comparability between hospital star ratings. Specifically, CMS proposes to:
If finalized, these changes will be used to calculate the Overall Star Ratings beginning in CY 2021.
Hospital OQR and ASC Quality Reporting (ASCQR) Programs
Key Takeaway: CMS proposes changes to align the Hospital OQR and ASCQR Programs.
For CY 2021, CMS proposes updates and refinements to the requirements for the Hospital OQR Program and the ASCQR Program. CMS is not proposing any measure additions or measure deletions in this rulemaking cycle.
CMS previously finalized that hospitals sharing the same CMS Certification Number must combine data collection and submission across their multiple campuses for all clinical measures for public reporting purposes. For CY 2021, CMS proposes to codify this policy by adding language to regulation text. CMS solicits comments on this proposal. CMS also proposes to codify an expanded review and corrections process to further align the Hospital OQR and ASCQR Programs while clarifying program requirements.
Finally, CMS seeks public comments on new measures for consideration that address care quality in the ASC setting, and on additional measures that could facilitate comparison of care provided in ASCs and hospitals.
For more information, contact Jennifer Archer, Paul Gerrard, Deborah Godes, Sheila Madhani or Jessica Roth.