The Transitional Add-on Payment Adjustment for New and Innovative Equipment and Supplies (TPNIES) is a recently enacted program by the Centers for Medicare & Medicaid Services (CMS) to drive innovation for new medical technologies in the end-stage renal disease (ESRD) setting. Implemented in 2020, the add-on payment addresses barriers present under bundled payment systems that fail to incentivize and pay for new technologies when first available to the market. CMS designed the TPNIES program similar to the New Technology Add-on Payment (NTAP) in the inpatient prospective payment system (IPPS); however, following such a framework may fail to reflect some of the important differences across the two payment systems as well as repeats many of the challenges already found with the NTAP program. We look at some of these challenges in more detail below.
LACK OF INNOVATION IN THE ESRD SETTING
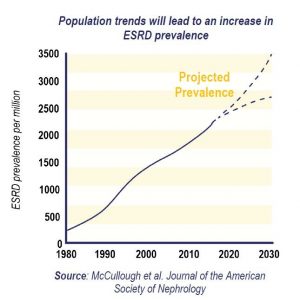
The lack of novel products is especially concerning as the prevalence of ESRD continues to grow in the US, impacting more and more Medicare beneficiaries. Nearly 600,00 individuals are on dialysis in the U.S.[v] Total Medicare spending on ESRD patients was in excess of $36 billion in 2018, which represents 7.2% of overall Medicare paid claims.[vi]
TPNIES Criteria and NTAP Comparisons
TPNIES has been adopted by CMS with the intent to encourage innovation for dialysis services and equipment and “facilitate beneficiary access to certain qualifying, new and innovative renal dialysis equipment and supplies”.[vii]
Both TPNIES and NTAP use specific regulatory criteria to evaluate and reimburse products, as compared in the chart below.
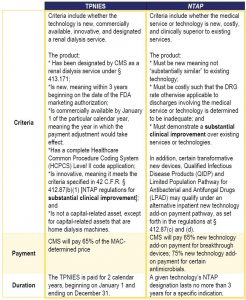
While both TPNIES and NTAP require a showing of substantial clinical improvement (SCI) to ensure the add-on payments are for “new equipment and supplies that are truly innovative,”[viii] CMS continues to set a high bar in evaluating products to meet this standard.
The SCI guidance does not appear as limiting as it highlights a number of ways to show such improvements and emphasizes that a product only needs to demonstrate one of the following outcomes[ix],[x]:
But when applied in practice, the provision of sufficient SCI evidence typically requires manufacturers to present stringent trial data and additional evidence. Products seeking the add-on payment in both settings must often dedicate additional resources and conduct time-consuming analyses beyond the data gathered for FDA approval in order to apply for the add-on payments. This becomes even more challenging as CMS has a historically low rate of approval for applications. Of 95 NTAP applications between 2003 and 2018, only 30% have been approved.[xi] Similarly, no device has been granted an approval for TPNIES (although the program has only been through one application cycle).
This dynamic is seen in the difficulty to meet the SCI criteria, which creates an even greater barrier for ESRD products. As noted previously, the lack of clinical trial enrollees and funding for research can complicate obtaining sufficient evidence. Specifically, CMS continues to seek very specific data on Medicare beneficiaries, limiting the evidence it will consider from applicants. This requirement also drives at the very problem TPNIES seeks to address—without the add-on payment, few US facilities are willing to adopt new products. Ultimately this results in challenges in securing sufficient Medicare beneficiary data before the product has broad adoption in the US.
CMS has noted that it will consider “the totality of the evidence” and a variety of sources, including “real world evidence and outcomes”.[xii],[xiii] Yet, when applied to the TPNIES applicants, CMS continued to stress the importance of more formalized randomized controlled trials despite other types of supportive evidence. In the last rulemaking evaluating the TPNIES applicant, CMS stated “[perceived clinical benefits] were not assessed in rigorously conducted, randomized clinical studies.” Both applicants were denied despite the showing of other clinical evidence supporting SCI criteria.
TPNIES creates a pathway for greater adoption of innovative and new products for ESRD patients that was not available until last year. Accordingly, manufacturers now have an opportunity and some incentive to put forward new products and test how CMS will move the program forward. On November 2, 2020, CMS released the final rule with one TPNIES expansion in eligibility “to include certain capital-related assets that are home dialysis machines when used in the home for a single patient.” This action to expand the benefit of this add-on payment may be indicative of future plans that align with overall kidney care payment goals. The next round of TPNIES applicants will be addressed in the ESRD CY 2022 proposed rule, which may outline changes in CMS’s thinking and approach to the program.
[i] Linde PG, Archdeacon P, Breyer MD, et al. Overcoming barriers in kidney health—forging a platform for innovation. Journal of the American Society of Nephrology. 2016:27(7), 1902-1910.
[ii] Palmer SC, Sciancalepore M, & Strippoli GF. (2011). Trial quality in nephrology: how are we measuring up?. American journal of kidney diseases: the official journal of the National Kidney Foundation, 58(3), 335-337.
[iii] Linde PG, Archdeacon P, Breyer MD, et al. (2016). Overcoming barriers in kidney health—forging a platform for innovation. Journal of the American Society of Nephrology, 27(7), 1902-1910.
[iv] Based on a review of FDA de novo approvals from 2015 -2020.
[v] National Kidney Foundation. (2021). Kidney Disease: The Basics. https://www.kidney.org/news/newsroom/factsheets/KidneyDiseaseBasics
[vi] The United States Renal Data System (USRDS). Healthcare Expenditures for Persons with ESRD. (2020). https://adr.usrds.org/2020/end-stage-renal-disease/9-healthcare-expenditures-for-persons-with-esrd.
[vii] 42 CFR 413.236. (2020).
[viii] 85 Fed. Reg. 71398 (Nov. 9, 2021).
[ix] CMS, Substantial Clinical Improvement Criteria for TPNIES, available at https://www.cms.gov/files/document/cy-2021-tpnies-substantial-clinical-improvement-criterion.pdf (emphasis added).
[x] 42 CFR 413.236(b)(5). (2020).
[xi] Truglio A, Livoti C. (2018). Analysis of success rates for the Center for Medicare and Medicaid’s new technology add-on payment program. Value in Health/ISPOR. 21(1). S117-S118.
[xii] 85 Fed. Reg. 71398 (Nov. 9, 2021).
[xiii] Number of randomized control trials in nephrology is the lowest of all internal medicine specialties: 2,779 in nephrology compared to 27,109 in cardiology (between 1966 to 2002).
*Baxter International Inc. provided financial support for portions of this newsletter.
For more information, contact Paul Gerrard, Kristen O’Brien, Jennifer Ohn or Paul Radensky.