The Trump Administration launched the American Kidney Initiative on July 10, 2019, when President Trump signed an Executive Order (EO) pledging transformation and innovation to improve care for kidney patients and reduce healthcare costs. Over the past year, the Administration has taken steps to implement the EO’s principles, including proposing new payment models, issuing regulations and making policy changes intended to facilitate and encourage organ transplant options. This +Insight reviews the actions of the past year and forecasts the work still to come.
Key Resources
The Kidney Care Landscape
The effects of kidney disease on the US healthcare system are significant. Across the United States, 37 million patients suffer from chronic kidney disease (CKD), and more than 700,000 have end-stage renal disease (ESRD). Traditional Medicare spends about 20% of its funds on individuals with kidney disease. Dialysis, the primary treatment for kidney failure, is burdensome on patients and costly to the system. A kidney transplant is the only permanent cure, but with almost 100,000 Americans currently on a waiting list, it is not a viable option for many individuals experiencing kidney failure. As a result, kidney disease remains the ninth leading cause of death in this country.

The Coronavirus (COVID-19) pandemic has further complicated kidney care and worsened outcomes for patients and caregivers. Like others with underlying health conditions, people with kidney disease are at higher risk of COVID-19-related complications, and traveling to dialysis centers increases the chances of exposure. Current data indicates that COVID-19 often affects the kidneys in addition to the respiratory system. The virus has been shown to impair renal function in a variety of ways. According to recently released preliminary COVID-19 data derived from Medicare claims, Medicare’s ESRD beneficiaries have the highest COVID-19 hospitalization rate, with 1,341 cases per 100,000 beneficiaries. Patients with ESRD are also more likely to have chronic comorbidities associated with increased COVID-19 complications, such as diabetes and heart failure, according to the Centers for Medicare and Medicaid Services (CMS).
Even prior to the release of the presidential EO, transforming kidney care was among the few areas of agreement between Republicans and Democrats in the health policy space. The EO focused attention on specific steps to accelerate solutions and new approaches.
EO on Advancing American Kidney Health
On July 10, 2019, President Trump issued an EO on Advancing American Kidney Health. This EO was a sweeping, widely supported initiative intended to improve the lives of Americans suffering from kidney disease and reduce healthcare costs.
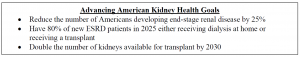
Since the release of the EO, the Administration has taken concerted steps towards these goals by:
While many of these actions have been underway pre-pandemic, COVID-19 created new challenges and has slowed the pace of reforms.
HHS Actions to Implement the Kidney EO
Payment Models to Encourage Home Dialysis and Transplantation
In tandem with the EO, US Department for Health and Human Services (HHS) Secretary Alex Azar and CMS Administrator Seema Verma announced five new payment models, one mandatory and four voluntary. The proposed mandatory ESRD Treatment Choices Model would encourage home dialysis and transplantation. Like other CMS Innovation Center models, it aims to achieve these objectives while reducing Medicare expenditures and preserving or improving quality of care for beneficiaries with ESRD. Under the proposed model, CMS would randomly select ESRD facilities and clinicians based on their location, equivalent to 50% of adult ESRD beneficiaries across the country. The model would apply a uniform positive adjustment to claims for home dialysis and related services, and a positive or negative adjustment based on home dialysis and transplant rates.
As with other mandatory models, the ESRD Treatment Choices Model faced some criticism, including from the Medicare Payment Advisory Commission, which expressed significant concerns about flaws in the proposed payment methodology and incentives for providers. Throughout both the Obama and Trump Administrations, CMS’s attempts to roll out mandatory models have been controversial. Supporters argue that mandatory models may be necessary to encourage participation by those that are unwilling to voluntarily adopt payment changes, and that voluntary models are vulnerable to selection biases that reduce savings. Critics argue that mandatory models overstep the appropriate role and authority for the Innovation Center, and that required participation can create burden for participants. In addition to the regular political challenges around mandatory models, the Trump Administration now faces the challenge of a global pandemic, which further complicates the political and stakeholder dynamics around all models, but especially mandatory ones that demand cost savings. The ESRD Treatment Choices Model was originally proposed to begin January 1, 2020. A final rule has not yet been issued.
The CMS Innovation Center also announced a series of payment options with the Kidney Care Choices (KCC) Model, which consists of the Kidney Care First (KCF) Model and the Comprehensive Kidney Care Contracting (CKCC) Graduated, CKCC Professional and CKCC Global options. These options are intended to build on the existing Comprehensive End Stage Renal Disease Care model and vary in the amount of risk and reward participants accept.
The KCF Model will provide participating nephrology practices with adjusted capitated payments for managing the care of patients with CKD Stage 4–5 or with ESRD. KCF practices will also receive incentive payments for successful transplantation.
The CKCC models aim to bring together dialysis facilities, nephrologists and other providers to manage care for ESRD patients. Like KCF, these options include increasing levels of financial accountability, including a full risk model. CKCC is aligned with other Innovation Center efforts, including Direct Contracting and Primary Care First, that aim to encourage a move away from fee-for-service payments and toward prepayment and streamlined performance metrics. The KCF model and the CKCC Professional and Global Models are expected to qualify as Advanced Alternative Payment Models under the CMS Quality Payment Program beginning in 2021. Specific details on all of the models have remained sparse.
In a series of announcements on Innovation Center models, CMS delayed the start of the first KCC performance period to April 1, 2021, and announced a second cohort that would begin January 1, 2022. The Innovation Center will now be in a sprint to fully roll out these models in advance of the first performance year.
ESRD and Medicare Advantage Payment Rules Encourage Innovation
The Administration has also taken regulatory steps to advance technological developments and encourage home dialysis.
For example, a proposal in the recently released 2021 ESRD Proposed Rule would increase support for home dialysis. In last year’s ESRD Prospective Payment System final rule, CMS finalized a policy to establish transitional add-on payment adjustments for certain new and innovative renal dialysis equipment and supplies (referred to as TPNIES). The intent of this add-on payment is to support beneficiary access to and ESRD facility use of new technologies. This payment adjustment, similar to the new technology add-on payment and transitional pass-through payment in the inpatient and outpatient setting respectively, adjusts payments to ESRD facilities as they incorporate new and innovative equipment and supplies into their businesses, and supports ESRD facilities transitioning or testing new products.
In the CY 2021 rule released in July 2020, CMS proposed application of the transitional add-on payment adjustment to home dialysis machines in certain circumstances. Specifically, CMS proposed that capital-related assets that are home dialysis machines when used in the home for a single patient would be eligible for this transitional payment adjustment, provided that the other criteria are met. Equipment that meets the criteria would be eligible for additional pass-through payment for two years equal to 65% of the pre-adjusted per-treatment amount for the machines. This proposed change is intended to encourage greater adoption of new technology and to stimulate innovation and investment in this space.
In June 2020, CMS also issued a final rule that will allow ESRD beneficiaries to enroll in Medicare Advantage (MA) plans beginning in 2021, implementing a statutory requirement of the 21st Century Cures Act. Most ESRD beneficiaries were previously prohibited from enrolling in MA plans.
The Cures Act also changed the payment rules so that MA organizations are no longer responsible for organ acquisition costs for kidney transplants for MA beneficiaries. Instead, those costs will be carved out from MA benchmarks and included in the fee-for-service program.
CMS also relaxed certain network adequacy requirements. MA plans may select their provider networks but are required to ensure that benefits are available and accessible to beneficiaries within the plan’s service area. CMS requires that plans meet access and availability standards, including maximum time and distance standards for member access to providers, including specialists. In the June 2020 final rule, CMS codified the list of provider and facility types that are subject to network adequacy reviews, requirements and exceptions. The final rule also implemented changes to take into account telehealth providers in contracted networks when determining compliance with network adequacy requirements. CMS stated that it will give MA plans credit toward these standards for certain provider specialty types when the plan contracts with telehealth providers for those specialties, including nephrology.
CMS also removed outpatient dialysis from the list of facility types to which the time and distance standards apply. Instead, MA plans are required to attest that they have enough dialysis services available for beneficiaries. In finalizing the policy, CMS stated that “this change will drive patient-centered treatment in dialysis services, which is at the heart of our intent in considering this change in policy.” CMS reiterated its belief that the policy will allow MA plans to enhance their networks with dialysis providers that offer home-based modalities, thus giving enrollees greater flexibility, encouraging greater competition and reducing costs for beneficiaries. A patient group recently filed a lawsuit asking the US District Court for the District of Columbia to find the policy unlawful and vacate it, citing concerns about administrative procedure and anti-discrimination violations.
Facilitating and Encouraging Transplantation
Kidney organ transplantation is considered the best therapy to extend life expectancy and quality of life for ESRD patients. Transplanted organs can come from deceased as well as living donors, yet the average wait time for a kidney can be three to five years, and even longer in some regions of the country. An estimated 20 Americans die each day waiting for an organ, and 83.1% of individuals on a transplant waitlist are waiting for a kidney. Recently, HHS has tried to increase availability by loosening restrictions and creating incentives and opportunities for transplantations.
In December 2019, CMS and the Health Resources and Services Administration (HRSA) released two proposed rules to increase the number of transplants in the United States. CMS released the Organ Procurement Organization (OPO) Conditions for Coverage Proposed Rule, which focused on improving the performance of OPOs. OPOs procure organs from hospitals and deliver them to transplant centers. Due to logistical problems and transport delays, every year thousands of organs (mainly kidneys) are wasted and must be discarded. OPOs act as the matchmakers responsible for everything from donor consent to delivering the kidney to the final destination. OPOs overall performance has been under scrutiny by CMS and stakeholders. OPO performance is based on self-reported data from measures that were last reviewed in 2006. CMS proposed a series of new measures and benchmarks, and proposed increasing the frequency of OPO reviews from every four years to every 12 months. CMS estimated that if all OPOs were to meet the new proposed standards, the number of annual transplants would increase from about 32,000 to 37,000 by 2026, for a total of almost 15,000 additional transplants in that time.
The other proposed rule, released by the HRSA, would expand the scope of reimbursable expenses for living donors to include lost wages and childcare and eldercare expenses for donors who lack other forms of financial support. HRSA currently provides for reimbursement of up to $6,000 in expenses, including travel, lodging, meals and incidental expenditures related to donor evaluation or other donation-related medical procedures.
In December 2019, HRSA also indicated that they are preparing a separate notice that would increase the income threshold for living donors eligible for reimbursements. The agency believes that there are many potential living organ donors who would like to donate an organ to a family member or friend, but cannot afford the loss of income or the loss of medical insurance benefits incurred during the weeks out of work required for the transplant surgery and recovery.
The comment periods for both of these proposed rules have closed, and we await the issuance of the final rules.
In early June 2020, HHS and the US Public Health Service published an updated solid organ transplant guideline to assess donors and monitor recipients for human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus infections. This new guideline will expand the pool of potential donors to include individuals who previously may have been declined because of concerns over possible HIV or hepatitis infections. This greater flexibility is possible because advances in highly accurate testing have made it easier for healthcare providers to quickly determine whether a potential organ donor has an infection.
While all of these actions seem aligned toward increasing the pool of donors, COVID-19 may have disrupted many facilities’ capacity to perform transplant procedures, and live donors may be less inclined to donate during a public health emergency.
Promoting the Development of an Artificial Kidney
The development of an artificial kidney has the potential to improve treatment options for patients and reduce healthcare costs. An artificial kidney could negate the need for burdensome weekly dialysis treatments and could serve as an alternative to donor organs, which are in short supply. The potential benefits and broad applicability of this envisioned technology have driven many private companies and academic institutions around the globe to pursue its development.
The EO on Advancing American Kidney Health promoted the development of an artificial kidney by calling for enhanced cooperation between developers and the US Food and Drug Administration (FDA), the agency responsible for the approval of medical devices. The EO also directed HHS to produce a strategy for encouraging innovation in new therapies through the Kidney Innovation Accelerator (KidneyX), a public-private partnership between HHS and the American Society of Nephrology. To this end, HHS issued a request for information to launch the Artificial Kidney Prize in conjunction with the presidential EO. The Department requested proposals for the development of an artificial kidney and for solutions to discrete problems currently facing the product development community. Winners are expected to be announced at the KidneyX summit on July 22, 2020.
The Administration’s encouragement of artificial kidney development is consistent with its approach in other areas where it has relied on public-private partnerships. An artificial kidney is a solution that may take time to come to fruition, however. While scientists have been working to develop this technology for more than a decade, a successful model may not come to market for some years yet. This timeline means that the development of an artificial kidney should not be included in any near-term strategy to address the quality and cost of care for kidney patients, even though it is a critical component of any long-term strategy.
The Future of Innovation in Kidney Care
The COVID-19 pandemic has highlighted many vulnerabilities in the healthcare system, including care for kidney patients. As noted, patients with kidney disease are at increased risk for serious complications from the virus. The exposure risks for kidney patients who must frequently visit facilities for inpatient care seem particularly acute.
While the final kidney payment model rules and organ-transplant-related rules are pending, the recent update to the solid organ transplant guideline seems to indicate that transforming kidney care remains a priority for the Trump Administration. The presidential election in November creates some uncertainty around the future of these initiatives, however, as with many programs and policies. While Republican and Democratic support for improving kidney care may keep this issue front and center regardless of the election outcome, specific approaches may vary depending on the leadership in the White House and at HHS.
For more information, contact Deborah Godes, Sheila Madhani or Mara McDermott.