McDermott+ is pleased to bring you Regs & Eggs, a weekly Regulatory Affairs blog by Jeffrey Davis. Click here to subscribe to future blog posts.
July 10, 2025 – The last week of June was a busy one when it comes to news about prior authorization – a major tool used by health plans and the federal government to manage healthcare utilization. The week started with a commitment from major health plans to reform their prior authorization processes and ended with the release of a new payment model from the Centers for Medicare & Medicaid Services (CMS) Innovation Center called the Wasteful and Inappropriate Service Reduction (WISeR) Model that will subject a set of services under Medicare fee-for-service (FFS) in specific geographic areas to prior authorization.
To help dig into the new model, I’m bringing in my colleagues Rachel Hollander and Simeon Niles. We’ll start with an overview of the model, given that prior authorization is a relatively new concept for Medicare FFS, then highlight three important considerations for stakeholders as they think about the model’s implications.
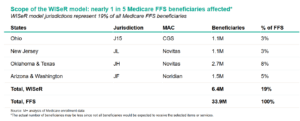
On June 27, 2025, the CMS Innovation Center released a press release, public notice, and official website for the WISeR Model, a six-year payment model aimed at reducing fraud, waste, and abuse in the Medicare FFS program. Beginning January 1, 2026, and running through December 31, 2031, the model will test technology-enabled prior authorization and pre-payment review to ensure that selected services are medically necessary and clinically appropriate. It will operate across six states (New Jersey, Ohio, Oklahoma, Texas, Arizona, and Washington) within four Medicare Administrative Contractor (MAC) jurisdictions (JL, J15, JH, and JF) affecting nearly one in five Medicare FFS beneficiaries. (Note: the actual number of beneficiaries may be less since not all beneficiaries would be expected to receive the selected items or services.)
Under the WISeR Model, CMS will contract with third-party technology vendors – the model participants. These vendors must have demonstrated experience using tools such as artificial intelligence, machine learning, and automated decision logic to manage prior authorization processes for other payers, including Medicare Advantage (MA) plans. Model participants will be responsible for reviewing claims for a predefined list of services that CMS believes are prone to overuse or improper billing, and will be compensated by CMS based on a percentage of demonstrated savings from avoided payments for selected services. Applications must be submitted by July 25, 2025, meaning that potential applicants have to move quickly.
Although providers and suppliers in the selected states are not formally considered model participants and may technically opt out of the prior authorization process, all Medicare-enrolled providers and suppliers will ultimately be subject to the model’s requirements. Claims for included services submitted without prior authorization will instead undergo prepayment medical review. In practice, provider/supplier participation will play out in one of three ways:
Providers and suppliers may resubmit prior authorization requests an unlimited number of times following a non-affirmation decision. They may also request a peer-to-peer clinical review during resubmission to address the reason for non-affirmation and discuss alternative clinical options. If a claim is submitted and denied after a non-affirmation decision, it will be subject to the standard Medicare claims appeals process. CMS will exempt certain providers/suppliers from prior authorization if they achieve a provisional affirmation rate of 90% or higher during a designated performance review period.
Takeaway 1: WISeR raises questions about services that have already gone through rigorous and comprehensive coverage decisions.
The CMS press release states that services targeted under the WISeR Model “provide little to no clinical benefit,” may “result in physical harm and psychological stress,” and are broadly characterized as “low value.” Although CMS labels the selected services in such manner, they all fall under national coverage determinations (NCDs) or local coverage determinations (LCDs), including some that are subject to coverage with evidence development requirements. Rigorous evidence reviews and established standards of care are required before CMS grants an NCD or the MACs grant an LCD. These services have also passed the US Food and Drug Administration’s “safety and effectiveness” thresholds.
While the CMS Innovation Center will not modify any NCDs or LCDs in this model, it will likely scrutinize the NCDs and LCDs associated with the selected services, as well as providers’ adherence to them, over the course of the model. Based on CMS’s evaluation of the model and whether it confirms CMS’s assertions about the services, the agency may decide to make changes to the LCDs and NCDs themselves. CMS could also decide to subject more services that fit under the categorization of low value and no clinical benefit to prior authorization going forward.
Takeaway 2: WISeR is classified as a voluntary model, but it is mandatory for providers and suppliers in selected states.
The CMS Innovation Center frames WISeR as a voluntary model because its “participants” are technology vendors that voluntarily apply to operationalize the prior authorization process. However, participation is functionally mandatory for all providers and suppliers in the selected states. Any claim for a selected service that is not preceded by a prior authorization request will automatically be subject to prepayment medical review, which introduces additional administrative processes, documentation requirements, and potential delays. This effectively imposes a new standard for providers in the selected states, regardless of their willingness to enter the model.
Mandatory models must go through formal notice-and-comment rulemaking to be established, but voluntary models can be implemented through informal announcements or notices. The CMS Innovation Center’s decision to classify this model as voluntary allowed the Center to circumvent the rulemaking process, which includes an opportunity for public comment and engagement, and move more quickly towards implementation.
Takeaway 3: WISeR may reflect a broader strategy to align FFS and MA oversight.
At first glance, WISeR may appear to contradict CMS’s ongoing efforts to streamline the use of prior authorization by private health plans, including within the MA program. As described in this Regs & Eggs blog post, CMS’s announcement of the new health plan commitment follows on other recent agency actions attempting to improve prior authorization processes for providers and patients. This model, conversely, adds prior authorization requirements to an area that traditionally hasn’t used this tool to control costs. While one could view this model as a reversal of CMS’s present and historical position on prior authorization, another perspective to consider is that the model represents an attempt to level the playing field between MA and FFS by bringing consistent administrative tools to coverage decisions in both programs. By introducing technology-driven prior authorization into FFS, CMS may be working toward a more uniform set of administrative rules across Medicare, so that providers are subject to the same accountability standards regardless of whether they treat a beneficiary enrolled in MA or in Medicare FFS.
Beyond our three takeaways, other facets of the WISeR Model may be worth exploring. Stay tuned for more as McDermott+ continues to dig into the details and possible implications of this new model.
Until next week, this is Jeffrey (and Rachel and Simeon) saying, enjoy reading regs with your eggs.
For more information, please contact Jeffrey Davis. To subscribe to Regs & Eggs, please CLICK HERE.