McDermott+ is pleased to bring you Regs & Eggs, a weekly Regulatory Affairs blog by Jeffrey Davis. Click here to subscribe to future blog posts.
May 1, 2025 – To state the obvious, a lot has happened in healthcare over the first 100 days of President Trump’s second term. But how much? Well, McDermott+ has been keeping track of the number of healthcare regs published; healthcare-related executive orders (EOs); memorandums and other actions; and Biden-era health regs that have been paused – and the total is 77. While 77 may not seem like a large number, the implications of these actions are far-reaching as they have touched most, if not all, of the millions of different healthcare stakeholders.
So, let’s break down the 77 actions and provide some highlights within each of the buckets.
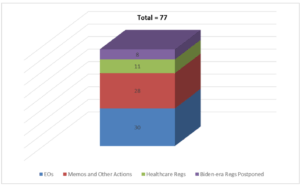
EOs: President Trump has put out 30 EOs that have had a direct or indirect impact on the healthcare sector. From his first healthcare-related EO that announced his intent to withdraw from the World Health Organization, to the recent EO on reforming higher education accreditation (including for medical schools), the EOs have covered a broad range of topics. Some of the major themes include:
EOs themselves usually do not effectuate policy, but rather lay out an administrative priority and provide a directive to a federal agency to take action on that priority. However, in many cases, President Trump’s EOs have laid out policy priorities that represent significant shifts from those of the previous administration (and one could argue that some lay out priorities that are different from the priorities of his first term in office). And because these EOs propose sweeping policy changes, they have created uncertainty, since many stakeholders are still assessing all the ways they could be impacted once the policies go into full effect. Federal agencies are in various stages of carrying out the EOs, and we will continue to monitor the administration’s progress on each of them.
Memos and Other Actions: The administration has issued 28 memos, policy statements, and other documents impacting healthcare. One could argue that these actions have had as large or even larger an effect on stakeholders as the EOs. We include in this bucket the US Department of Health and Human Services’ announcement and efforts around restructuring the department. Some of these actions carry out EOs, including guidance around reductions in force to reshape the federal workforce, memos and requests for information on deregulation, and memos following up on the “Protect Children from Chemical and Surgical Mutilation” EO.
New Regs Published: While deregulation has been a major priority in this administration, that hasn’t stopped federal agencies from issuing 11 healthcare-related regs thus far. Five of these regs represent the annual Medicare proposed payment regs for facilities, including the fiscal year 2026 Inpatient Prospective Payment System proposed reg. The first reg issued was a Centers for Medicare & Medicaid Services proposed reg related to integrity and affordability within the Affordable Care Act Marketplaces. Comments were due on April 11, 2025, so this reg has a chance to be the first to go through the full rulemaking process (proposed reg, comment period, and final reg) under this administration. Not counted in our total are about half a dozen healthcare-related regs currently being reviewed by the Office of Management and Budget that could be released over the next several months.
Biden-Era Regs Postponed: We knew from the first days of President Trump’s second term that he planned to review President Biden’s regs and potentially pause those that had not yet gone into effect. In total, the administration has paused eight regs that were released under Biden. Two of those regs relate to use of buprenorphine to virtually treat patients with opioid use disorder. The US Drug Enforcement Administration initially proposed to delay the effective date of the final rules titled “Expansion of Buprenorphine Treatment via Telemedicine Encounter” and “Continuity of Care via Telemedicine for Veterans Affairs Patients” to March 21, 2025, but after receiving public comments, ultimately delayed the regs’ effective date to December 31, 2025. The delay effectively means that the current telemedicine flexibilities that have been in place since early in the COVID-19 pandemic will continue through the end of the calendar year.
Numbers definitely don’t tell the entire story here, and we have provided in-depth updates and analyses through +Insights, Regs & Eggs blog posts, and other documents on McDermott+’s First 100 Days Resource Center. Although the first 100 days of this administration have come and gone, rest assured that we will continue tracking major actions and discussing how they affect providers, payers, patients, and other healthcare stakeholders.
Until next week, this is Jeffrey saying, enjoy reading regs with your eggs.
For more information, please contact Jeffrey Davis. To subscribe to Regs & Eggs, please CLICK HERE.